Implementing Flow Meters Significantly Improves Fab Process Quality

Reproducibility and maintaining strict quality control throughout the entire wafer manufacturing process are top priorities in the semiconductor industry. Thus, stable and highly accurate flow measurement is essential for many semiconductor manufacturing steps. Non-contact clamp-on flow meters such as SEMIFLOW CO.65 have proven to fulfill this demanding task reliably.

The ultrasonic sensors measure through the wall of the tube or pipe and substantially reduce the risk of contamination or leakage of hazardous fluids. With the SEMIFLOW flow meter series, the ultrasound specialist SONOTEC has developed metal-free contactless flow meters particularly designed for the requirements in the semiconductor industry – also for hazardous environments. The compact sensors with integrated electronics board are available in multiple sizes suitable for all common rigid plastic tubes and pipes used in fabs.
Non-Contact Flow Monitoring in CMP Processes
Reproducibility and maintaining strict quality control throughout the entire wafer manufacturing process are top priorities in the semiconductor industry. Thus, stable and highly accurate flow measurement is essential for many semiconductor manufacturing steps. Non-contact clamp-on flow meters have proven to fulfill this demanding task reliably. The ultrasonic sensors measure through the wall of the tube or pipe and substantially reduce the risk of contamination or leakage of hazardous fluids. With the SEMIFLOW flow meter series, the ultrasound specialist SONOTEC has developed metal-free contactless flow meters particularly designed for the requirements in the semiconductor industry – also for hazardous environments. The compact sensors with integrated electronics board are available in multiple sizes suitable for all common rigid plastic tubes and pipes used in fabs.
In order to guarantee continuous high product quality, a tight liquid flow monitoring is necessary. From liquid storage containers to the application in the process, the liquid flow must be monitored and tracked, e.g., to measure the volume flow in a transfer line or to control the volume output at the points of use. Ultrasonic clamp-on flow meters can be implemented in low- and high-volume manufacturing settings and used reliably regardless of the type or blending of the chemicals in the tube.
Reliable Monitoring of Slurry Consumption in Chemical-Mechanical Planarization
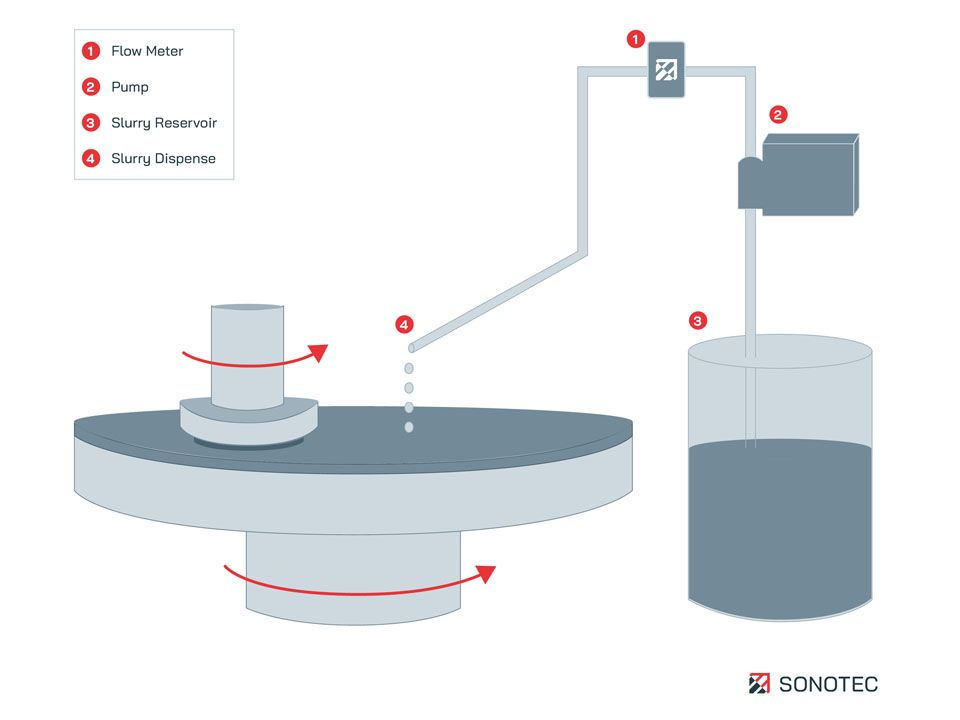
Simplified schematic illustration on a chemical-mechanical planarization (CMP) process with implemented non-contact flow meter
Chemical-mechanical planarization or polishing (CMP) is one of the leading technologies to manufacture state-of-the-art microelectronic components and micro-electro-mechanical systems (MEMS). In order to produce modern integrated circuits with multiple wiring levels and smallest line widths, highly precise flat and smooth surfaces are needed. CMP processes have become also very important in the finishing of wafer-thinning processes and the production of vertical contacts through the silicon wafer.
Since the industry is persistently looking for further ways to reduce the structure width, the requirements in process design are becoming more detailed and stringent. Hence, CMP processes are getting more complex and quality standards grow constantly. As a consequence, process parameters have to be monitored very closely at various steps to avoid deviations in product quality that might lead to costly failures.
In CMP processes, flow meters accurately monitor the dispensing of slurry on the polishing plate. Additionally, they are installed to count the volume flow to calculate the amount of slurry used at this process step. Flow sensors also monitor precisely the consumption of slurry in supply tanks or in the pipe system to determine the transfer volume. Every fab uses its own slurry blending. Non-contact flow meters are well-suited for contamination-free flow or volume measurement of liquids, work independently of the slurry composition and are free of wear and tear.
Fundamentals on Chemical-Mechanical Polishing / Planarization (CMP)
CMP is a key technology in any semiconductor fabrication. It is applied in both, substrate as well as device fabrication. Advanced semiconductor devices easily need more than 30 CMP processes to get to its final stage.
CMP aims on perfect smooth surfaces with almost no remaining topography. To that, the wafer surface is polished using a slurry and a polish pad. Slurry describes a chemical fluid that contains abrasive nanoparticles dispersed in acidic or basic solution. During the CMP process step the wafer is pressed on the rotating polish pad, while slurry is continuously dispensed. Specifically, the slurry chemically modifies the uppermost surface layer, which is then mechanically cracked and removed by slurry abrasives.
Efficient Slurry Blending and Dispensing at Each Point of Use
Large semiconductor manufacturing sites commonly implement slurry mixing and blending systems to ensure optimal slurry concentration for the specific process requirements. For the slurry preparation, the different components are mixed with DI-water. With regard to achieve exactly the predefined slurry concentration at the point of use, highly accurate flow meters monitor the volume flow rate of the components from buffer tanks to the mixing container. This real-time control with contactless flow sensors ensures proper blending of the chemicals at high production rates and specific mixing ratios.
Automatic slurry dispense platforms guarantee an efficient slurry distribution during continuous operations in CMP manufacturing environments. In order to have a redundant system monitoring, non-contact flow meters are installed in addition to each distribution pump to make sure that the defined product quality remains constantly at the same high level. Contactless ultrasound flow measurement guarantees that the slurry or chemical in the pipe or tube is not contaminated or influenced by the measurement device. Additionally, the flow sensor can conveniently be replaced or moved to another position without having to intervene in the closed pipe system.
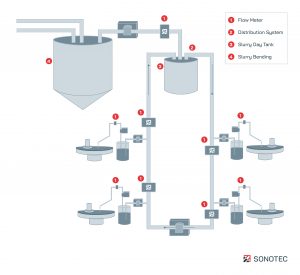
Simplified schematic illustration on slurry management and slurry distribution with implemented non-contact flow meter
Slurry Management and Distribution
By monitoring the slurry composition and concentration with automatic dosing and blend correction throughout the entire pipe system, precise metrology options are used. Via additional tank or mixing stations in the fab, blend corrections can be processed. For this step, highly accurate and reliable flow meters control the related pumps. Thus, the exact slurry blending can be ensured and efficiently used. Additionally, flow meters guarantee both system and component redundancy throughout the entire wafer manufacturing process.
In large manufacturing facilities, transfer pumps control the slurry supply via fab-wide loop pipe systems. For redundancy reasons, implemented clamp-on flow sensors monitor the liquid flow or volume in the pipe system.
Precise Distribution of Photoresist in Lithography Applications
In semiconductor manufacturing, the process of photolithography describes the processing of circuit board designs on silicon wafers by means of light. The process starts with coating the wafer with a chemical layer called photoresist. The layer of the photoresist must be extremely precise. For the exact volume dosing of these photoresist layers, highly accurate flow meters are implemented to redundantly monitor the pump behavior. By implementing non-contact ultrasonic SONOCHECK air bubble detectors, even smallest air bubbles can be detected that might occur in the coating process.
In a further step, light passes through a photomask and creates an optical image of the circuit layout on the wafer. The generated light reacts with the photoresist which is washed away in the next process step. Thus, the underlying oxide layer is exposed. By using certain acid baths, further oxide and residues of the underlying silicon layer are removed. As the acid baths have to be kept with an even filling level, non-contact flow meters precisely monitor the filling of the wet benches. Thanks to the non-contact measurement method it is not necessary to replace the sensor after cleaning procedures or servicing. The described process is repeated several times with different combinations of chemicals and masks, building up the layout structure of the later processor.
Finally, the doping process is applied to the exposed silicon, changing its electrical properties. Current photolithography processes often include around 30 or more separate masks to layer circuit patterns on top of each other.
Image © SONOTEC GmbH


 The Autosamdri®-931, the 5th generation automatic Critical Point Dryer (CPD) from Tousimis’ Autosamdri® range, has recently been installed at one of Germany’s most prestigious research universities. A technical university with a clear mission to advance science and technology for the benefit of society. Progress and success can only be achieved with the highest quality equipment.
The Autosamdri®-931, the 5th generation automatic Critical Point Dryer (CPD) from Tousimis’ Autosamdri® range, has recently been installed at one of Germany’s most prestigious research universities. A technical university with a clear mission to advance science and technology for the benefit of society. Progress and success can only be achieved with the highest quality equipment.